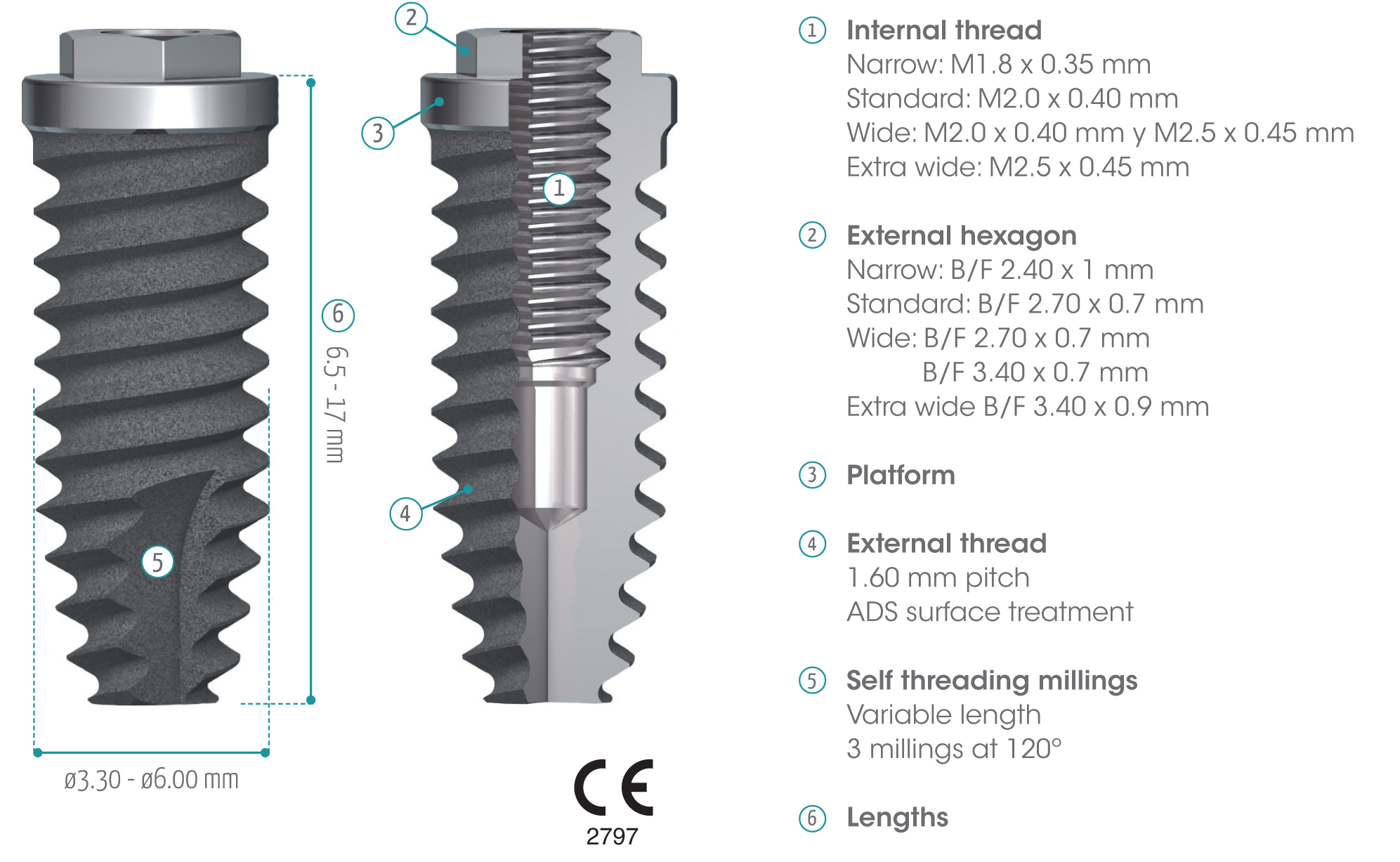
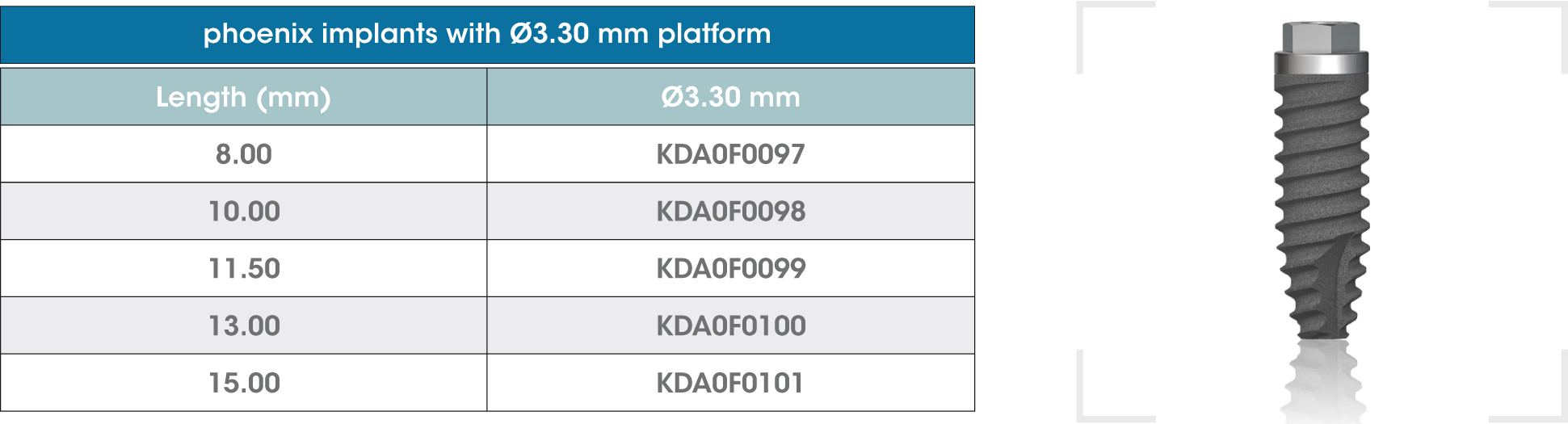
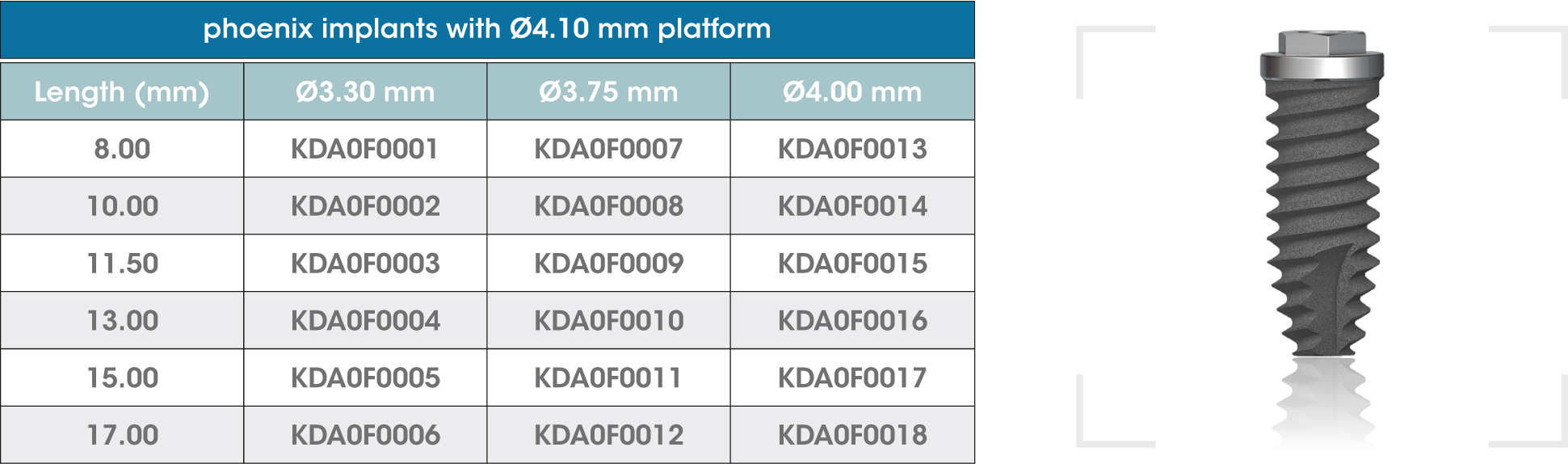
gmi phoenix external connection implants are machined in CP grade IV titanium of the highest quality, thus ensuring a high chemical stability and excellent biocompatibility. The titanium oxide layer, that is formed on the implant surface after the passivation process, and the careful design of the outer surface of the thread, guarantee good osseointegration and high stability.
gmi phoenix external connection implants
The external dual inlet thread of the implant helps to reduce surgery time. Moreover, the rounded shapes, self-threading millings and slight tapering facilitate implant insertion, reducing tension at the bone-implant interface and thus preventing threading stress-related problems.
ADS® surface treatment
To promote the adhesion and growth of bone cells, the outer surface of the implant has been treated with the exclusive ADS® treatment (Advanced Double-Grip Surface) which combines a white corundum micro-bubble treatment and acid etching, for a non-smooth roughness that maximizes the contact surface between implant and bone and therefore improves stability and adhesion between them.
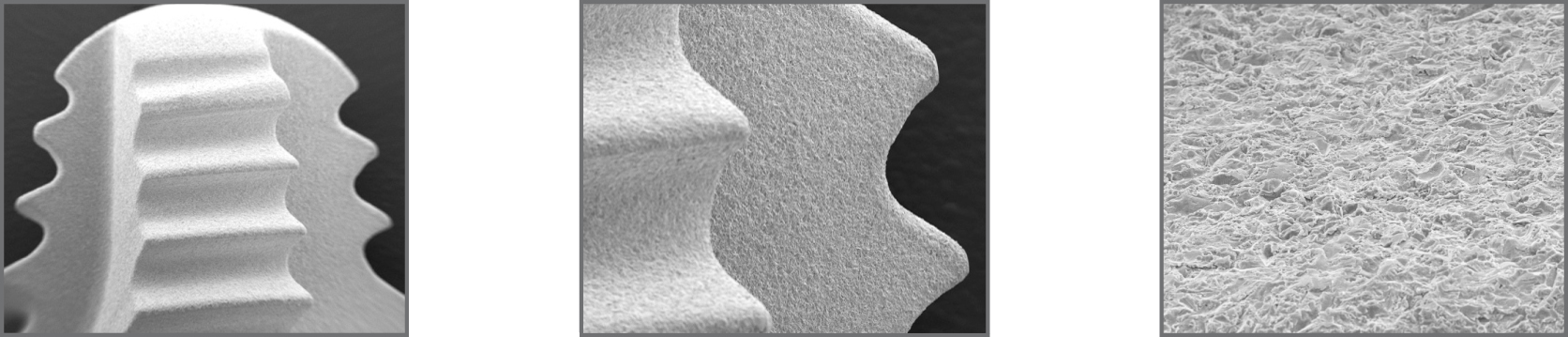
The ADS® treatment is the result of a series of collaborations with institutes and universities, as well as our own research and tests, that allow us to monitor and validate the optimal biological response to the implant surface: chemical (X-ray scattering studies to find out the composition of the implant and its surface) and topography studies (roughness studies) have been carried out, as well as biological (in vivo response studies in animals) and clinical studies.
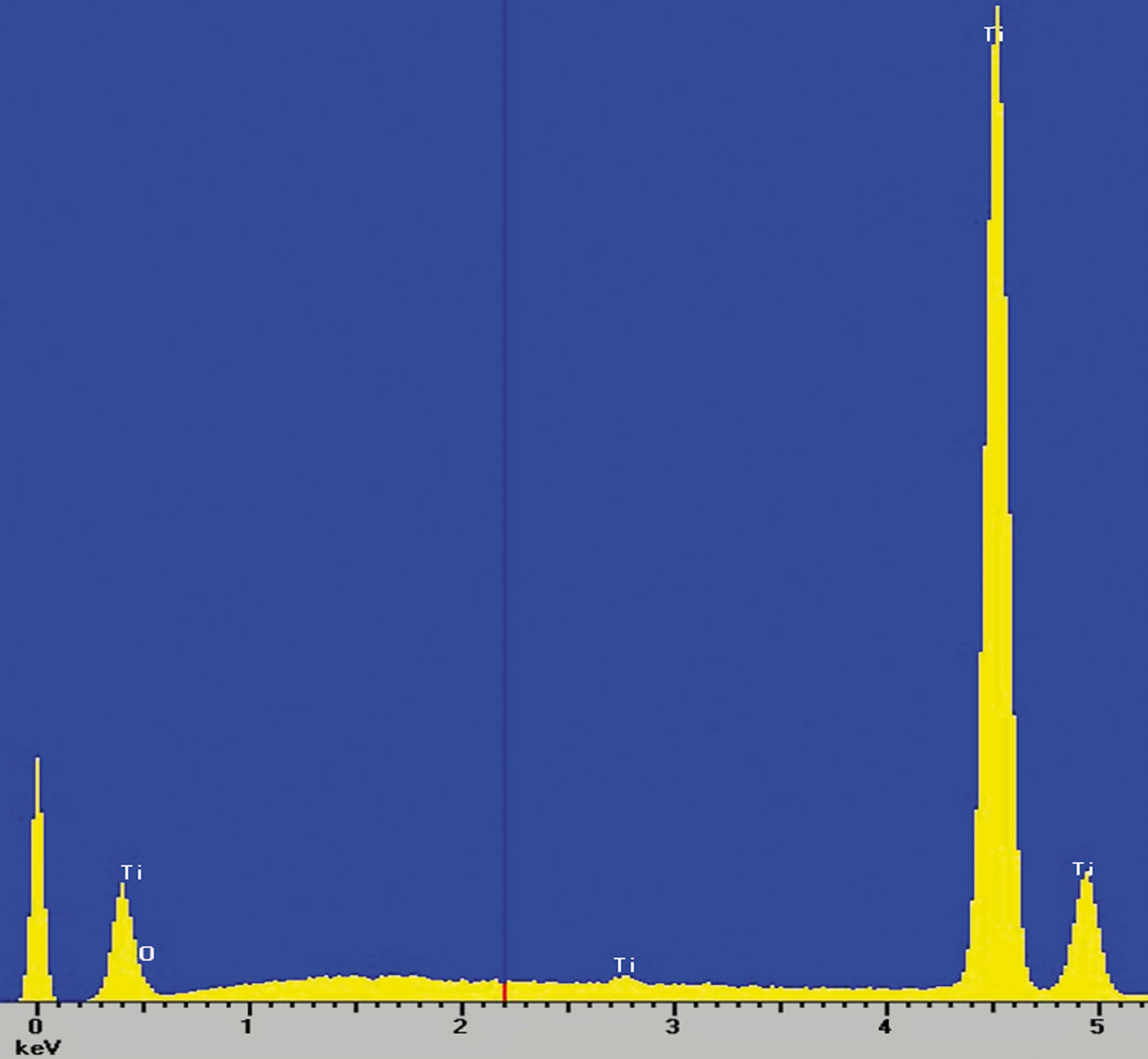
Composition studies
X-ray scattering studies show that the surface of the implants is only made of titanium and oxygen, which are the basic components of the oxide layer that is formed in the implant passivation process and that gives it its excellent corrosion resistance.
 |
Surface topography studies
In order to control and identify the topography of the implant surface, roughness studies were performed with profilometer, as well as with techniques such as SEM (Scanning Electron Microscope) and CLSM (Confocal Laser Scanning Microscope), allowing to obtain and control the mean roughness (Ra) values within the parameters recommended in international publications.
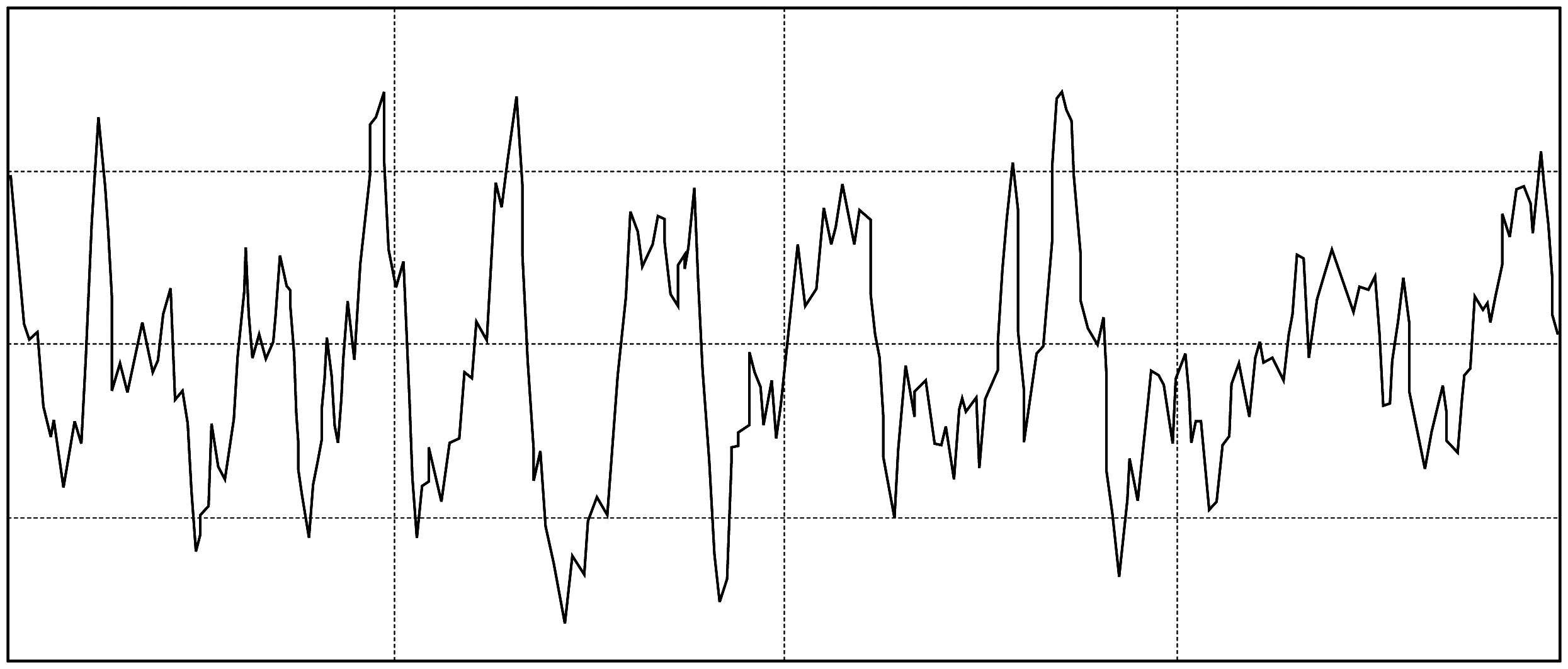
Roughness profile obtained with HOMMEL-WERKE T1000.
Osseointegration studies
To determine the biological response of gmi implants, in vivo studies were conducted by inserting the implants on animals, leaving them without load during the healing process and performing a histological study to prove the excellent response of the bone cells and the complete osseointegration of the implant.
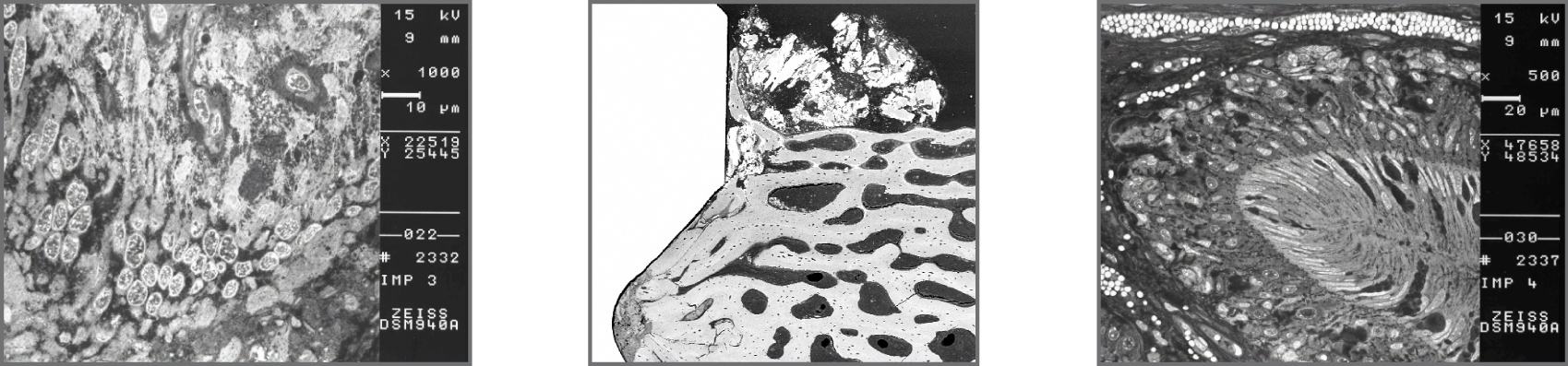
SEM-BSE: Ultrastructural details of cells in biofilm on the implant.
gmi phoenix external connection implants are packed in a screen-printed cardboard box, with three traceability labels and a double packaging containing the implant system, with the primary and secondary Pyrex glass packaging to ensure the inert performance of the container. Once packaged in a clean room, they are sterilized with gamma radiation according to regulations. Instructions for use can be consulted electronically.
The new packaging system of the gmi phoenix implants consists of a titanium support which only houses the implant-implant carrier set, prepared to be inserted directly into the mouth, using a single hex-3.00 mm implant carrier wrench, designed for this purpose. This new system offers the odontologist the following advantages: more control and a better view of the implant insertion process, it is easier to use in reduced interdental spaces and a reduction in surgery time.

* Consult the instructions for use in electronic format.
1. Check sticker indicating implant diameter and length and open the tab on the carton.
2. Remove the secondary packaging and product identification labels.
3. Check the integrity of the safety seal and unscrew the plug on the secondary packaging.
4. Remove the primary packaging from the secondary packaging avoiding hitting it against a hard surface.
5. Extract and save the primary packaging plug making a lateral movement.
6. Insert the HEX-3.00 mm implant carrier wrench until a slight retention is noticed, addressing the notches on the key with the faces of the implant hex broaching.
7. Check that the key is fully inserted and turn slightly while gently pulling on the assembly.
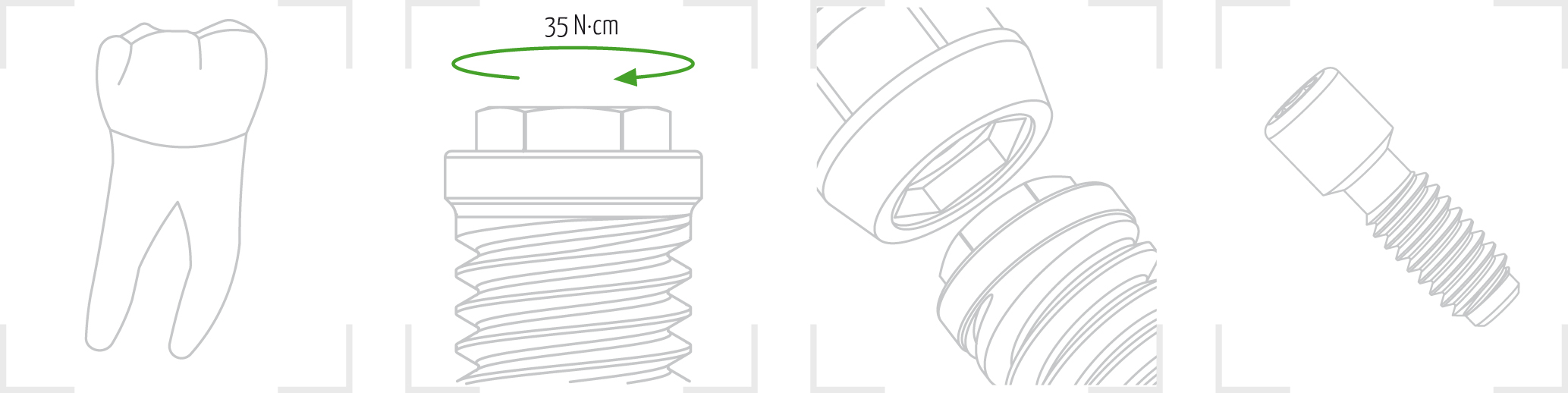
8. Place the implant in the bone bed by applying a maximum torque of 35 N·cm until the treated area is at the crestal or subcrestal level. Remove the key.
9. Unscrew the clinic screw and dismount the implant carrier by turning slightly if necessary.
10. Remove the cover screw from the primary packaging plug, using the HEX-0.80 mm ratchet wrench for Ø3.30 platform and HEX-1.20 mm for the other platforms.
11. Screw the cover screw to the implant by applying a maximum torque of 15 N·cm. Remove key and suture incision.
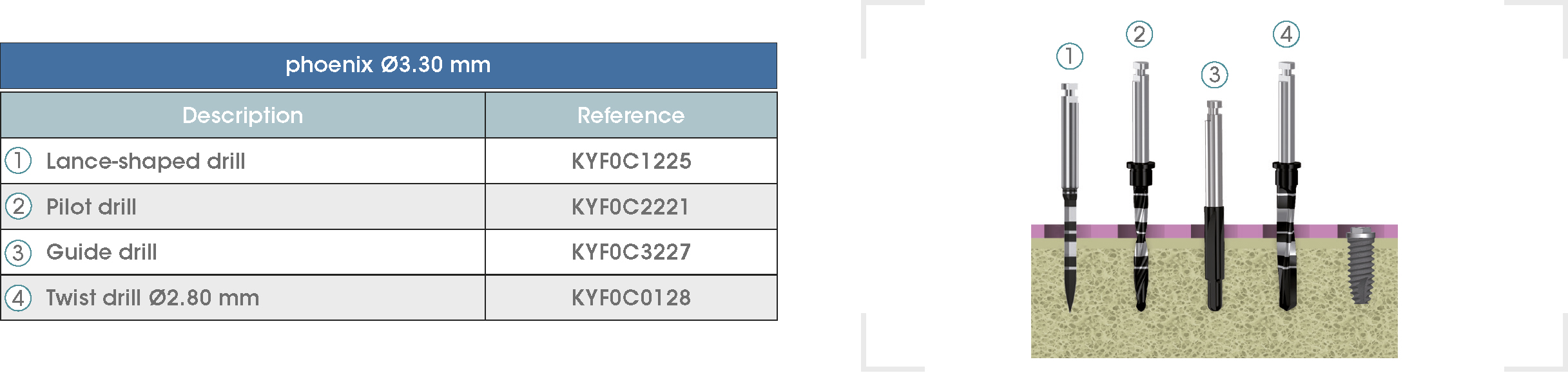
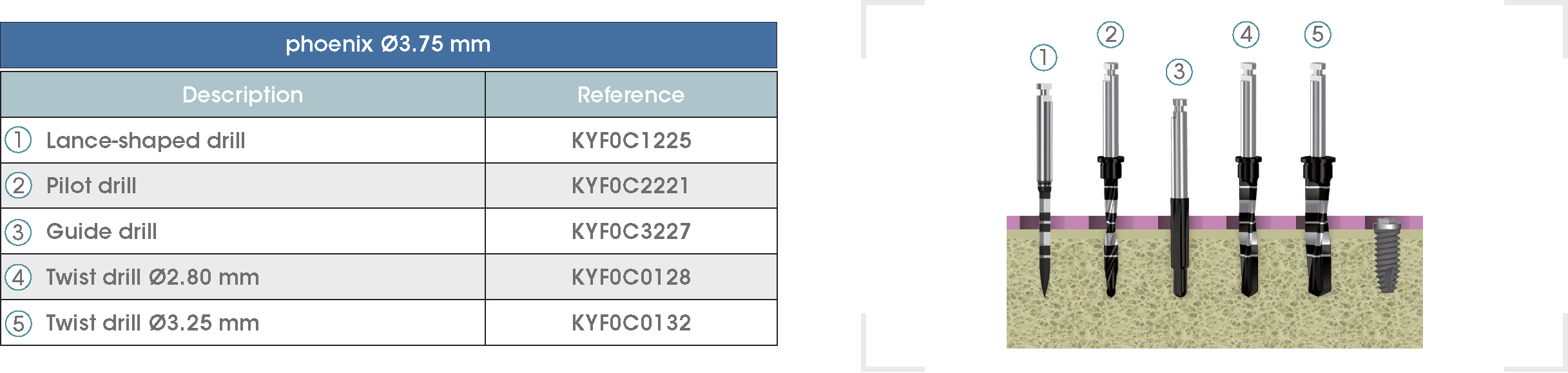
Following is a description of the drilling sequences for the different external connection implant models, as well as the recommended drilling conditions for their use.
• Lance-shaped: 1200 - 1500 rpm.
• Pilot drill: 900 - 1200 rpm.
• Guide drill: 800 rpm.
• Twist drills:
- Ø2.80 mm: 500 - 700 rpm.
- Ø3.00 and Ø3.50 mm: 400 - 700 rpm.
- Ø4.25 mm: 400 - 600 rpm.
- Ø5.10 and Ø5.40 mm: 300 - 500 rpm.
• For drilling, external cooling with saline solution is required.
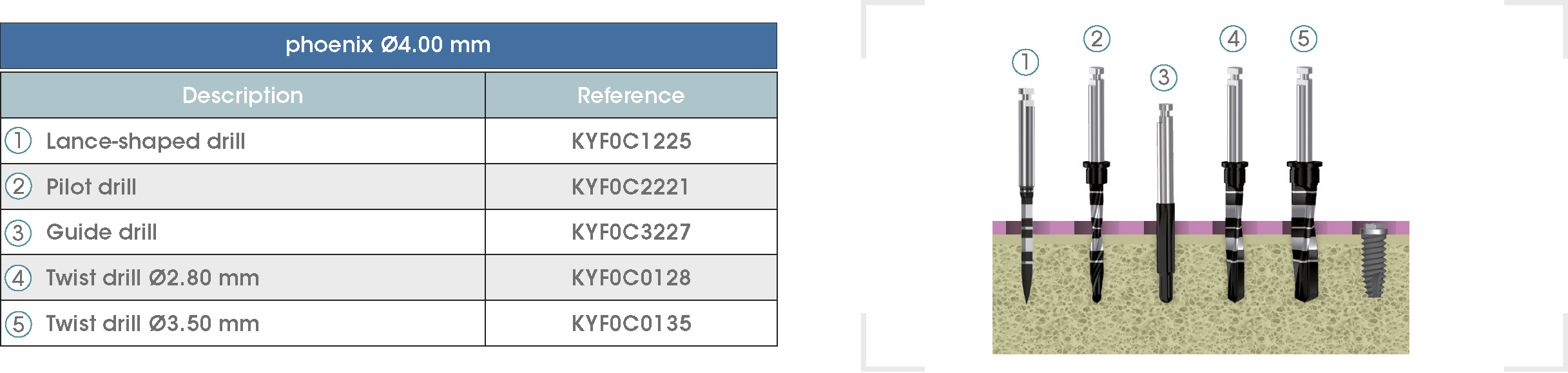
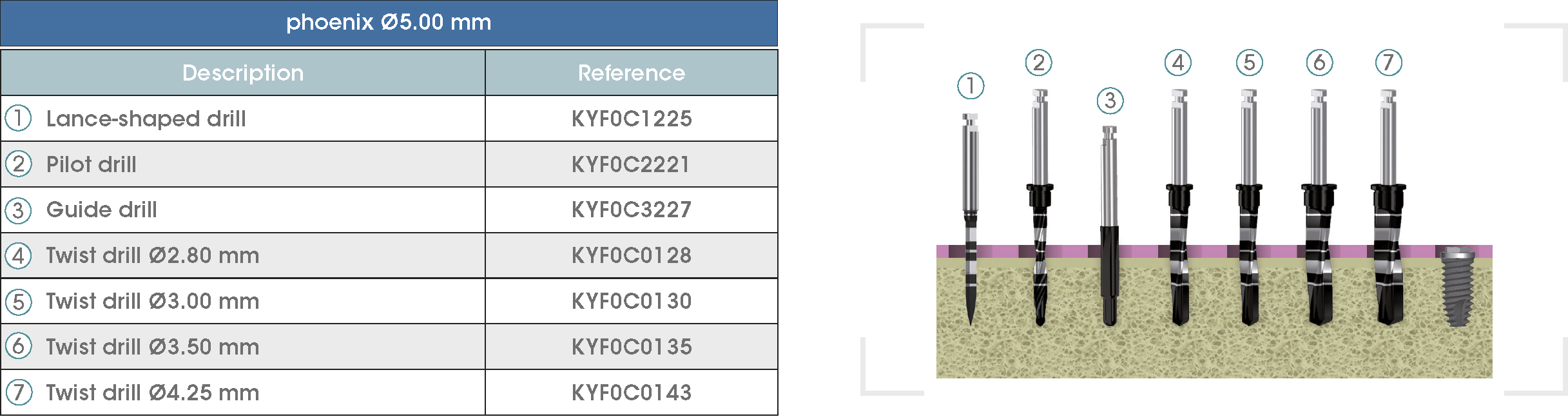
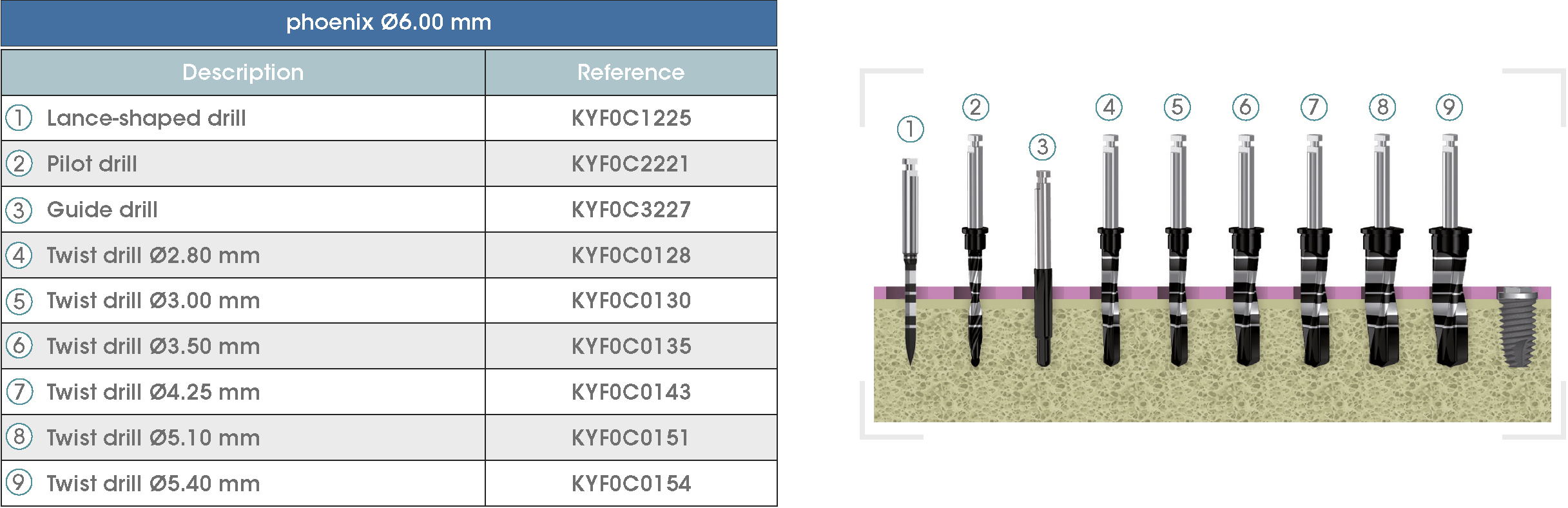
* Procedure recommended by GMI cannot replace the judgment and the experience of the surgeon.
The gmi phoenix implant range was designed and tested to be inserted as a single restoration, with a 30-degree maximum inclination to the perpendicular plane to the occlusal plane, in accordance with the following diagram.
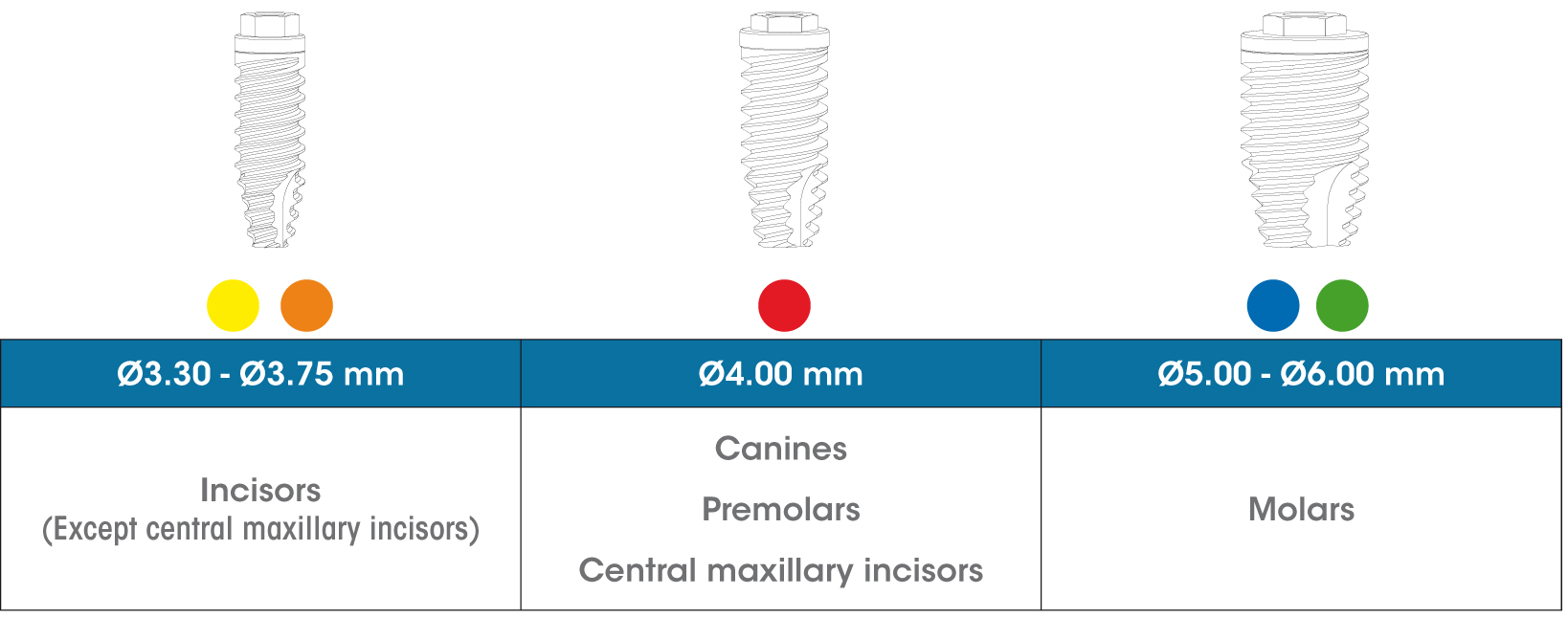
The gmi phoenix implant range is designed to be inserted applying a 35-40 N·cm torque, although it can bear a maximum tightening torque of 60 N·cm. GMI recommends manual insertion of the implant with a dynamometric ratchet wrench to ensure that these values are not exceeded.
The gmi phoenix range of implants and prosthetic attachments are designed and manufactured with tolerances intended to maintain adjustments that minimize the clearance between components and therefore the stability of the connection. gmi recommends the use of original attachments to ensure a perfect fit between the components of the restoration.
To prevent prior deformation of the clinic screw, gmi recommends restricting its use only for the final tightening of the prosthesis. To make adjustments during the prosthetic procedures, use of the laboratory screw is recommended.
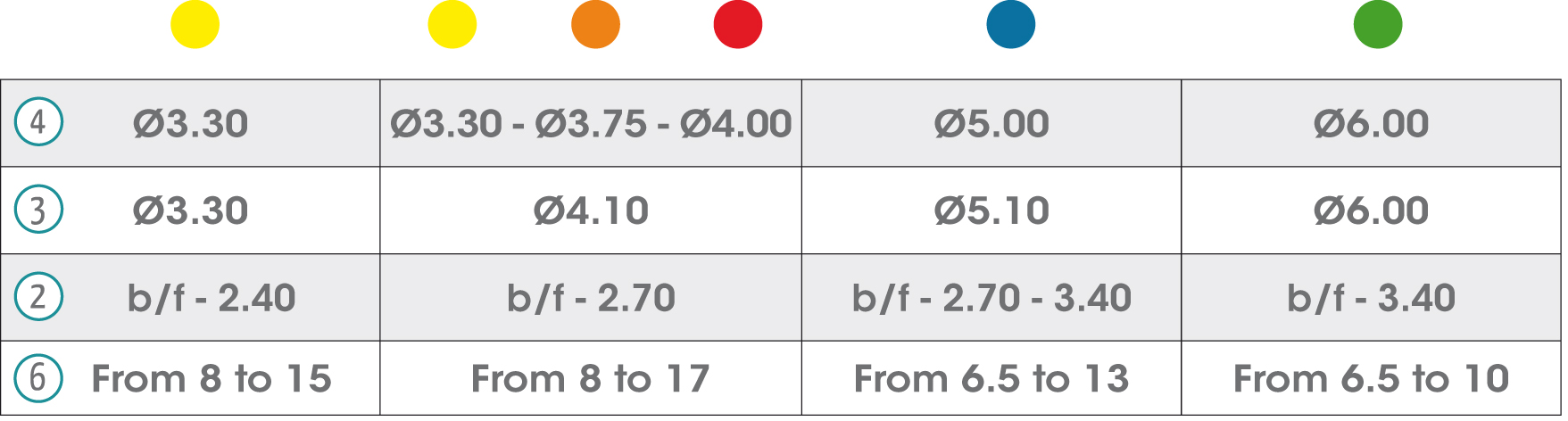
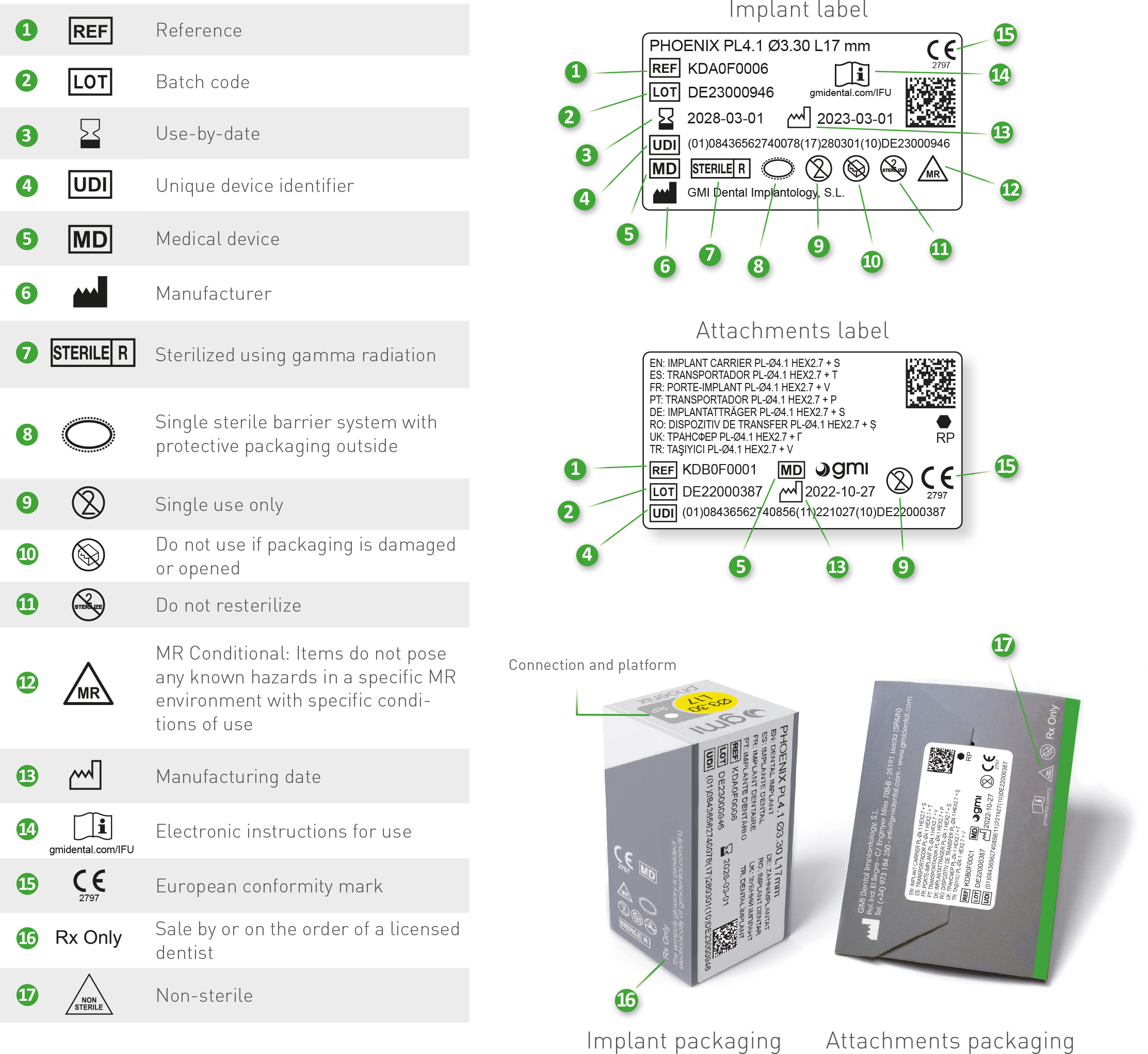
In the chart are detailed all the symbols appearing on the gmi phoenix implant system's labelling and packaging and their corresponding description.
DataMatrix specifications of labels:
